Abstract
Introduction: Minimal residual disease (MRD) assessments are being increasingly used to define prognosis in patients with acute myeloid leukemia (AML). However, data suggests that a proportion of patients found to be MRD negative experience leukemia relapse. These relapses are thought to be related to the persistence of leukemia stem cells (LSC) that survive chemotherapy. Recent studies have identified a number of LSC antigens that may be useful to follow LSC populations including CLL1, CD123 and CD47. We hypothesized that the use of a routine antibody panel in conjunction with CD123 and CLL1 specific antibodies will allow for more accurate determination of MRD using multiparametric flow cytometry (MPFC) and better identification of patients destined to relapse.
Methods: In this single center prospective study we evaluated the diagnostic bone marrow samples of all newly diagnosed patients with AML (excluding APL) for leukemia associated immunophenotypes (LAIP's) using MPFC and a panel of antibodies targeted towards myeloid, lymphoid and LSC markers. LAIP's were scored as a percentage of the bulk blast population which was defined using plots of Side Scatter (SCC) vs. CD45. LSC compartment was defined as CD34+CD38-CD123+ or CD34+CD38-CLL1+. Bone marrow samples from non-leukemic patients were used to identify the background level of CD123 and CLL1 positivity. Samples were considered MRD positive if having >0.1% of a specific LAIP as a function of the bulk blast population.
Results: Over the study duration, 88 patients with newly diagnosed AML received intensive chemotherapy (Table1). Complete MRD data (routine and LSC markers) was available for 58 patients had at the end of induction and 31 patients at the end consolidation (EOC). For the 58 patients with end-of-induction (EOI) MRD data the diagnostic sample revealed CD123+ LSC and CLL1+ LSC at a frequency of >0.1% in 30 (51.7%) and 28 (48.3%) patients respectively and at a frequency of <0.1% in 24 (25.9%) and 26 (44.8%) patients respectively. Data was not available for 4 patients.
Following a single induction chemotherapy cycle 52/58 (89.7%) patients were in complete remission. At the EOI, 26/58 (44.8%) patients were found to be MRD negative using the standard antibody panel. Only 3/30 patients (10%) who had CD123+ LSC and 2/28 patients who had CLL1+ LSC at diagnosis were found to be negative at the EOI. However, 21/24 (87.5%) patients that were negative for CD123+ LSC and 22/24 (85%) patients that were negative for CLL1+ LSC at diagnosis were found to be positive for CD123+ LSC and CLL1+ LSC at the EOI, respectively. At the EOC, 31 patients were assessed for routine and LSC based MRD. Amongst these patients 16 (51.6%) were MRD negative using routine markers, 13 (41.9%) were negative CD123 and only 6 (19.4%) were negative for CLL1.
There was no relationship between relapse rates and the MRD status at the EOI or EOC using either standard MRD markers, CD123 or CLL1. Although not statistically significant we observed that only 1 of 6 (16.7%) patients that was negative for CLL1+ LSC at the end of consolidation relapsed in contrast to 11 of 25 (44%) patients that were positive for CLL1+ LSC (p=0.4).
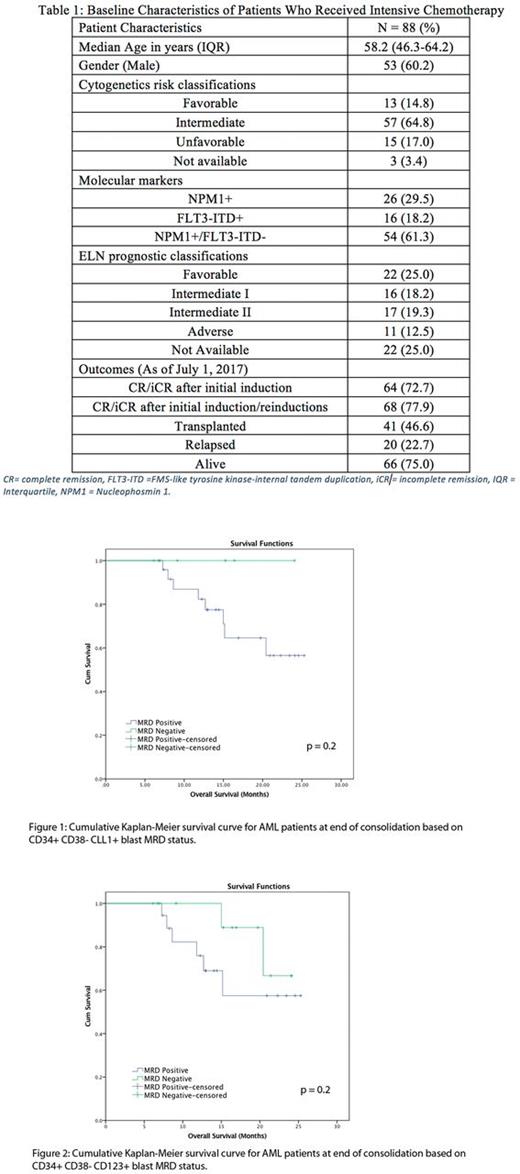
After a median follow-up of 11.3 months, the median relapse free survival for patients that were positive for CLL1+ LSC at the EOC was 13.8 months and was not achieved for patients negative for CLL1+ LSC (p=0.4). Similarly, there was no significant difference in the RFS for patients that were MRD positive or negative for routine MRD markers (16.8 vs. 13.8 months, p=0.94) or for CD123 (13.8 vs. 16.8 months, p=0.73). The median overall survival (OS) for the entire cohort was not achieved. There was no significant difference in the OS for patients based upon MRD status at the EOC when using standard MRD markers, CLL1 (Figure 1) or CD123 (Figure 2).
Conclusions: This proof-of-concept study suggests that the CLL1+ and CD123+ LSC can be identified at diagnosis at very low levels relative to the bulk blast population. Surprisingly, patients who were negative for CD123+ and CLL1+ LSC at diagnosis were often found to be positive at EOI. This may be related to a reduction in the overall blast population due to chemotherapy, but persistence of the original LSC population. Larger studies are necessary to explore the impact of persistent LSC markers on clinical outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal